Sex Hormones and Fat Distribution Patterns
Understanding how androgens and estrogens influence regional adiposity and sexual dimorphism in body composition.
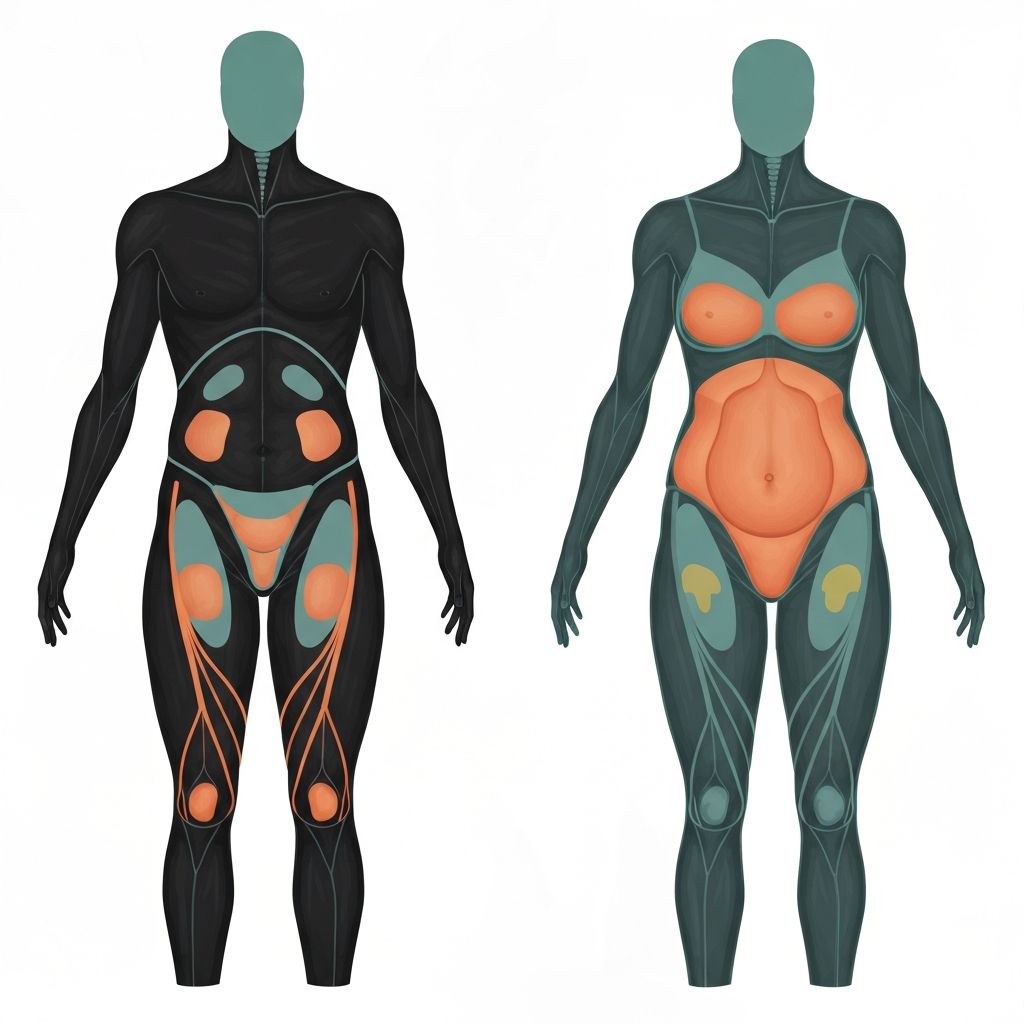
Sexual Dimorphism in Body Composition
One of the most striking physiological differences between biological males and females involves fat distribution patterns. Across all adult populations, sexual dimorphism in adiposity localization reflects the distinct influences of sex hormones on adipocyte development and regional fat storage. Biological females characteristically accumulate fat preferentially in subcutaneous compartments and gluteal-femoral (hip) regions, while biological males preferentially store abdominal fat, particularly in visceral depots.
This sexual dimorphism becomes apparent during puberty, when sex hormone production increases dramatically and sculpts sex-specific body composition patterns. Prior to puberty, males and females demonstrate relatively similar body fat distribution. During and following puberty, hormonal influences progressively establish the characteristic adult patterns.
Androgenic Effects on Fat Distribution
Androgens—primarily testosterone in males and small amounts in females—exert several effects on adipose tissue distribution and metabolism. Testosterone suppresses subcutaneous adiposity, particularly in the gluteal-femoral region, while promoting visceral and abdominal fat deposition in males. This androgenic direction of fat storage contributes to the characteristic male pattern of central adiposity.
Mechanistically, androgens achieve this distribution pattern through androgen receptor signaling in adipose tissue. Subcutaneous adipose tissue, particularly in lower-body regions, expresses high levels of androgen receptors. Testosterone signaling in these depots suppresses differentiation of preadipocytes into mature adipocytes, reducing subcutaneous fat accumulation. Simultaneously, visceral adipose tissue responds to androgens with enhanced metabolic activity and lipid storage.
Estrogenic Effects on Fat Distribution
Estrogens produce opposing effects to androgens on fat distribution. Estrogens promote subcutaneous fat accumulation, particularly in gluteal-femoral regions, while suppressing visceral fat deposition. Prepubertal females receiving estrogen therapy demonstrate accelerated development of characteristic female fat distribution patterns.
Estrogen receptor signaling in adipose tissue influences lipogenic gene expression and preadipocyte differentiation patterns differently across regional depots. Lower-body subcutaneous adipose tissue responds to estrogens with enhanced lipoprotein lipase activity and increased fat storage capacity. Visceral adipose tissue, conversely, demonstrates suppressed lipid storage under estrogenic influence.
Menopause and Postmenopausal Adiposity Shifts
The transition through menopause, accompanying marked decline in ovarian estrogen and progesterone production, produces substantial shifts in female fat distribution. Postmenopausal women demonstrate accelerated visceral fat accumulation and central adiposity increase compared to premenopausal counterparts despite stable body weight.
Longitudinal studies tracking women through the menopausal transition document consistent increases in visceral adiposity volume during the perimenopause and early postmenopause years, with acceleration particularly during the final menstrual transition period. These changes occur independent of weight gain, reflecting fundamental shifts in fat distribution mechanisms under altered hormonal conditions.
Interestingly, although postmenopausal women shift toward more central fat distribution (becoming more "male-like" in pattern), they do not fully adopt the male distribution pattern, retaining some preferential lower-body fat storage.
Andropause and Male Aging
Men experience progressive testosterone decline across adult aging, averaging approximately 1% per year from age 30 onward. This gradual androgen reduction associates with progressive increases in abdominal and visceral adiposity in aging males. Cross-sectional and longitudinal studies demonstrate that men with lower testosterone concentrations accumulate more central fat, even after adjustment for age and baseline fatness.
Testosterone replacement therapy in older hypogonadal men associates with reductions in total body fat and particularly visceral adiposity in many (though not all) intervention studies. These observations suggest that testosterone decline contributes to age-related central fat accumulation.
Hormonal Interactions and Complex Regulation
While sex hormones fundamentally influence fat distribution, they do not act in isolation. Androgens and estrogens interact with insulin signaling, cortisol effects, and adipokine production to shape overall fat distribution patterns. An individual's unique genetic makeup, combined with complex hormonal interactions, determines their specific fat distribution trajectory.
For example, the metabolic consequences of menopause partly reflect direct loss of estrogen's suppressive effects on visceral fat accumulation, but also involve estrogen's roles in insulin sensitivity, inflammatory regulation, and metabolic rate. Similarly, andropause involves both direct testosterone loss and secondary changes in metabolic function.
Regional Adipose Tissue Receptor Expression
The distinct effects of sex hormones on regional fat distribution reflect differential expression of hormone receptors across adipose tissue compartments. Androgen receptors show regional variation, with different expression densities in subcutaneous versus visceral depots. Estrogen receptors similarly demonstrate regional differences in density and subtype distribution.
These variations in receptor expression help explain why the same hormone concentrations produce different effects on different fat depots—an adipose tissue depot with high receptor density responds more sensitively to hormonal signaling.
Clinical Observations and Individual Variation
While general patterns of sex hormone effects on fat distribution are well-established, substantial individual variation exists. Some women with high testosterone concentrations may develop more central fat patterns. Some men with genetic predispositions may preferentially accumulate subcutaneous fat despite adequate testosterone. Genetic factors substantially influence sex hormone sensitivity and the ultimate expression of fat distribution patterns.
Educational Note
This article explains physiological mechanisms linking sex hormones to fat distribution patterns. Individual responses to hormonal changes vary substantially based on genetic factors, baseline metabolic status, and numerous other variables. This information is educational only and does not constitute medical advice or hormone recommendations.